- Previously retained placenta
- Multiparity
- Maternal age > 35 years
- Induction of labour
- Preterm labour
- Placenta Previa / abnormally invasive placenta
- Uterine anomalies eg. Bicornuate uterus, fibroids
- Previous uterine surgery or instrumentation
Retained Placenta Management (552)

Objectives
The aim of this guideline is to standardise management of retained placenta in order to minimise harm to the patient and reduce the risk of associated PPH.
| Please report any inaccuracies or issues with this guideline using our online form |
Retained placenta is defined as a placenta that remains in the uterus 30 minutes after active management of the third stage or 60 mins if management of the third stage is conservative .This occurs in 2-3% of all deliveries and is a risk factor for post partum haemorrhage (PPH). Haemorrhage, infection and genital tract trauma are recognised complications of the management of retained placenta.
Associated GGC policies:
- Full Bladder
- Constriction ring
- Morbidly adherent placenta
- Detached cord
- Uterine anomaly
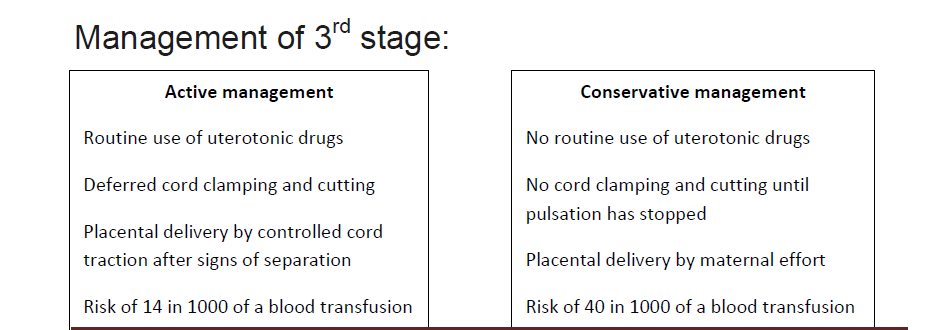
If the placenta is undelivered after 30 minutes of active management / 60 minutes ofconservative management and patient stable with no significant bleeding
- Do not leave woman unattended
- Regular maternal observations : pulse, blood pressure, respiratory rate every 15minutes
- Position change to upright
- Empty the bladder. If cannot pass urine then catheterisation should be carried out
- Encourage breastfeeding or nipple stimulation
- Call Obstetric specialty trainee earlier if concerns regarding bleeding or becomes haemodynamically unstable
Conservative management only : at 60minutes if placenta not delivered give 10iu IM syntocinon and wait a further 30 minutes if no active bleeding.
If undelivered by 45 minutes active / 90 minutes conservative
- Call Obstetrics Specialty Trainee to review and inform labour ward co-ordinator
- Regular maternal observations : pulse, blood pressure, respiratory rate every 15minutes
- Site IV access - large bore 16G grey cannula, and obtain FBC/ G+R
- Commence IV fluids - 1000ml crystalloid ( Compound sodium lactate (Hartmanns) solution or Sodium Chloride 0.9%)
- If bleeding consider syntocinon infusion (40iu syntocinon in 500ml sodium chloride 0.9% or compound sodium lactate at 125ml/hr). Do not start routinely as may make MROP more difficult.
- Accurate weighed estimated blood loss should be documented as a running total.
Obstetric Specialty Trainee:
- Offer vaginal examination
- Examination in the room is appropriate with verbal consent and if signs of separation have occurred, providing analgesia is adequate. Be prepared to abandon attempts and move patient to theatre if there is active bleeding or patient discomfort.
- Use of umbilical vein uterotonic drugs is no longer recommended
- Obtain informed consent for theatre
- Inform anaesthetist and theatre staff
- If there is significant delay in transfer to theatre consider indwelling catheter and cross match
Consent should include:
Risks of bleeding, infection, trauma to uterus or cervix, failure to remove all tissue, blood transfusion, repeat procedure, balloon insertion, laparotomy and hysterectomy.
- Surgical pause and review of consent
- Ensure analgesia is functional
- Aseptic technique - clean and drape
- IV Antibiotic cover - 1.2g of co-amoxiclav OR clindamycin 600mg IV + gentamicin
- Empty bladder
- Stabilise fundus with non dominant hand
- Gently insert hand through cervix and identify the placental plane
- If plane between placenta and uterus not easily defined consider placenta accreta and inform on call consultant. Do not pull on cord or placenta.
- Using the side of your hand and a sweeping motion sweep the placenta from the uterine wall
- Guard the fundus to avoid uterine inversion
- Grasp the uppermost portion of the placenta and aim to remove the whole placenta in one piece
- Check the cavity is empty - if in doubt call obstetric consultant on call
- Massage fundus
- Inspect for tears and repair as required
- IV syntocinon infusion should commence ( 40iu of syntocinon – 500ml of Compound sodium lactate over 4hours if active bleeding has occurred)
- Document in notes and debrief patient when appropriate
- DATIX to be completed if PPH or other complications
- Use of PPH section in notes to be completed where appropriate.
Patients who have had a retained placenta should be advised that all future deliveries should occur in an obstetric led unit as they have a higher risk of post-partum haemorrhage.
Conservative management may be considered for up to 180minutes in the absence of bleeding or shock. However if no signs of separation have occurred within 60minutes then manual removal may be appropriate.
